Carbohydrate wordle in the shape of a bread slice.
Monthly Archives: February 2013
Carbohydrates Multiple Choice
Hey guys, so you’ve read all the blogs and watched all the videos…so now its time to test yourself and see how much you’ve learnt! i have faith in you! let’s do this!
- Sucrose is composed of what monomers?
a) Glucose + Glucose
b) Fructose + Glucose
c) Cellulose + Glycogen
d) Glucose + Sucanose
2. What is a glycosidic bond?
a) Carbon from carboxylic acid bonded to Nitrogen from nitrile group
b) Disulphide Bond formed by two cysteine molecules
c) Hydrogen bond
d) Oxygen bridge formed by two hydroxyl groups
3. Another name for Lactose Intolerance is?
a) Lactosa Intolerosa
b) Lactate Malnutrition
c) Lactogen Deformation
d) Carbohydrate Malabsorption
4. What causes acid and gas formation of Lactose Intolerance?
a) Intestinal Bacteria
b) Fermentation
c) A series of reduction reactions
d) A series of oxidation reactions
5. What type of linkage does Amylose have between C1 + C4?
a) Beta 1 –> 6
b) Alpha 1 –> 6
c) Alpha 1 –> 4
d) Beta 1 –> 4
6. What is a “reducing end”?
a) anomeric C1 not involved in a glycosidic bond.
b) opposite end where redox reaction takes place
c) opposite end of anomeric carbon
d) None of the above
7. In Amylopectin, branches form after how many residues?
a) every other residue
b) 24 glu residues
c) 25 glu residues
d) branches randomly occur
8. What is an advantage of branching?
a) It makes the molecule more complex and hence stable
b) provide multiple chain ends at which enzymatic cleavage can occur
c) No advantage, branching just naturally occurs
d) To create redox ends.
9. What are the branches involved in Glycogen?
a) Beta 1 –> 6
b) Alpha 1–> 4
c) Alpha 1 –> 6
d) Beta 1 –> 4
10. Which dissacahride would give a negative result in Fehling’s Solution?
a) Glucose
b) Sucrose
c) Fructose
d) Galactlose
Disaccharides,Polysaccharides and Lactose intolerance
Welcome, welcome all! Let’s continue talking about carbohydrates shall we?! ..No worries, we’re almost through with it.
Today we shall discuss:
- Glycosidic Bonds
- Disaccarides
- Polysaccharides
- Lactose intolerance
- Tests for reducing sugars
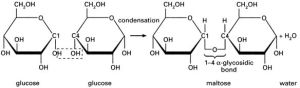
Glycosidic Bonds:
1) Two hydroxyl groups next to each other
2) One combines with a Hydrogen atom from the other to lose H2O molecule (condensation)
3) An oxygen bridge is formed, this is the glycosidic bond, keeping the two molecules together forming a disaccharide.
4) Glycosidic bonds can be broken by hydrolysis (addition of water).
Disaccharides:
Glucose + Glucose= Maltose
Glucose + Galactose= Lactose
Glucose + Fructose= Sucrose
All this talk about Glucose…
Polysaccharides:
Composed of monosaccharides, joined by condensation, that may be up to several thousands of units long.
Starch. Glycogen. Cellulose.
Starch: Storage in plants.
Composed of alpha 1 –> 4 linkage between Carbon1 and Carbon4 and Amylopectin. Amylopectin composed of alpha 1 –> 4 linkage and branches at every 24 glucosyl residues formed by 1 –> 6 linkage.
Reducing end: end of polysaccharide with anomeric Carbon1 not involved in glycosidic bond.
Advantage of glucose storage in polymeric form: reduces osmotic effects.
Advantage of branching: produces a compact structure and provide multiple chain ends at which cleavage can occur.
Glycogen: storage polymer in animals.
Similar to amylopectin except more highly branched (alpha 1–>6 branch at every 10 glucosyl residues)
Advantage of being highly branched: allows rapid release of glucose from glycogen stores.
Cellulose: Plant cell walls.
Beta 1–>4 linkage with every other glucose rotated 180 degrees.
Advantage of rotation: promotes intra-chain and inter-chain Hydrogen bonds and van der Waals interactions which causes cellulose to have a high tensile strength.
Now that those are covered, last but not least, let’s talk about Lactose Intolerance…that feeling in your stomach when you had roti and slupigen for lunch:
As persons grow older, lactase production decreases. therefore, more lactose is consumed that can be digested, that is, not enough lactase is present. Hence, lactose builds up in the stomach, and it attracts water, which accounts for bloating and diarrhea. Also, intestinal bacteria feed on undigested lactose, producing acid and gas.
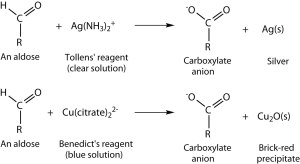
Tests for reducing sugars:
Positive result of Tollens reagent:
Fehling’s or Benedict’s solution on various sugars:
On that note…i bid you goodbye until next time (:
Hope you learnt something or got a better understanding of Carbohydrates!
References:
tumblr_m9wy7wyCkS1rohkrh
tumblr_m9r9gmVc5O1rohkrh
tumblr_m9r9lqSAxJ1rohkrh
tumblr_m9fuo4WImV1rohkrh
Carbohydrates!
Hello my fellow Biochemists! I’ve been attending carbohydrates lectures and tutorials for the past two weeks, and today I’d like to share what i have learnt with you.
Topics covered includes:
- Biosignificance
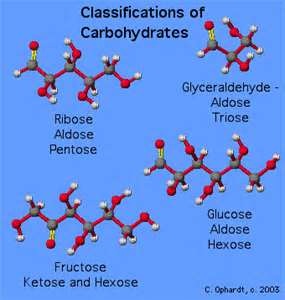
- Monosaccharides
- Isomerism
- Hemiacetal and Hemiketal
- Sugar Derivatives
Let’s get started!
First and foremost, carbohydrates are made by photosynthesis and ar essentially hydrates of carbons.
Photosynthesis Eq’n: Carbon Dioxide + Water + Light = Glucose + Oxygen
Functions of Carbohydrates:
- Energy Source
- Storage
- Structure
- Precursor molecule
Energy: respiration provides energy by breaking down carbohydrates to water and carbon dioxide.
Storage: plants use starch as storage while animals use glycogen as storage. Excess glucose is converted to glycogen by insulin while excess glycogen is converted to glucose by use of glucagon.
Structure: Carbohydrate cellulose makes up plant cell walls and provides its rigid structure. Cellulose is a polymer of Beta-D-Glucose. Chitin is another carbohydrate used for structre and it makes up the exoskeleton of insects.
Precuresor Molecules: for synthesis of certain biochemicals e.g ribose form part of the skeletons of nucleic acids DNA and RNA.
I know what you’re thinking..simple stuff huh? Now let’s get into the juicy stuff!
Isomerism:
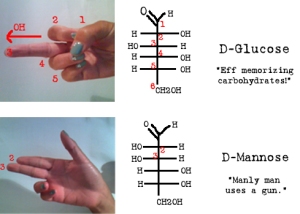
Chiral carbon are carbons with four different side or R-groups attached to it. Chiral carbons have mirror images but is not super-imposable.
D and L designations are based on the configuration about the single asymmetrical carbon, for sugars with more than one chiral centre, D or L refers to the asymmetrical carbon farthest from the aldehyde/ketone group. When the ‘OH’ is on the right hand side its refered as “D” and when its on the left hand side its refered as “L”.
Fischer and Haworth Projections:
- Epimers: Two sugars that differ only in the configuration around one carbon atom.
- Anomers: β and α Configuration. If the ‘OH’ is below the plane of the anomeric carbon then it is α (Alpha), and if the ‘OH’ is above the plane, it is Beta.
Difference between Epimers and Anomers:
Epimers differ in chirality at only one carbon. In the straight-chain form, epimers will have H and OH-substituents switched at one backbone carbon, but not at any others.
Anomers are special epimers; in cyclic forms of one single monosaccharide, anomers differ in chirality at the anomeric carbon only. In the straight-chain format, anomers will have the same configuation.
- Sugar Derivatives:
Sugar Alcohol: lacks an aldehyde or ketone.
Sugar Acids: Aldehyde at C1 or OH at C6 is oxidised to a carboxylic acid.
Amino Sugar: amino group substitutes for a hydroxyl. The amino group may be acetylated.
I hope this helped!
Cell Rap Vids!
Biochemists are truly the cool kids of the science world! so much rap videos on youtube about various biochem topics..i couldn’t resist posting them on my blog! i hope you enjoy them as much as i did.
Cells Quiz
Sit back, relax, and take the quiz!
1) Which cell feature is responsible for providing energy?
a.Lysosome
b.Mitochondria
c.Endoplasmic reticulum
d.Nucleus
2) Where does replication of DNA take place in the cell?
a.Golgi Apparatus
b. Ribosome
c.Nucleus
d.Nucleolus
3)Which is not a feature of prokaryotes?
a.Cell Membrane
b.Cell Wall
c.Endoplasmic Reticulum
d.DNA
4)Which structure serves to package,process and export proteins in the cell?
a. RER
b.Nucleus
c.Golgi Apparatus
d.Mitochondria
5) What is the phenomenon below?
a.Apoptisis
b.Enosymbiotic Theory
c.Phagocytosis
d.None of the above
6)What part of the cell manufactures proteins?
a.Ribosomes
b.Nucleus
c.Mitochondria
d.Cytoplasm
7)What feature is responsible for storing water?
a.Lysosome
b.Vacuole
c.Cytoplasm
d.Cell Wall
8)Why is the term ‘nuclear envelope’ used instead of ‘nuclear membrane’?
a. either term is correct
b.the enclosure is composed of two membranes
c. DNA membrane as well as nucleus membrane is considered
d. All of the above
9)Which cell feature transports ribosomes?
a.Cytoplasm
b.ER
c.Golgi Apparatus
d.Chlorophyll
10) Name the structure below:
a. Chloroplast
b.Golgi Apparatus
c. Circular DNA
d. White blood cell
Cells Wordle
Wordle based on Cells topic in the shape of a Red Blood Cell.
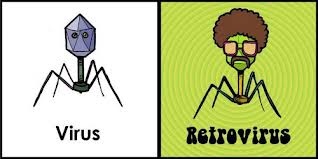
Remember this virus?
Cells!
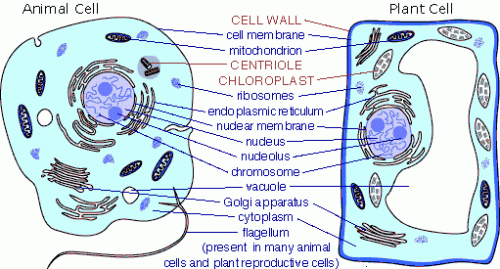
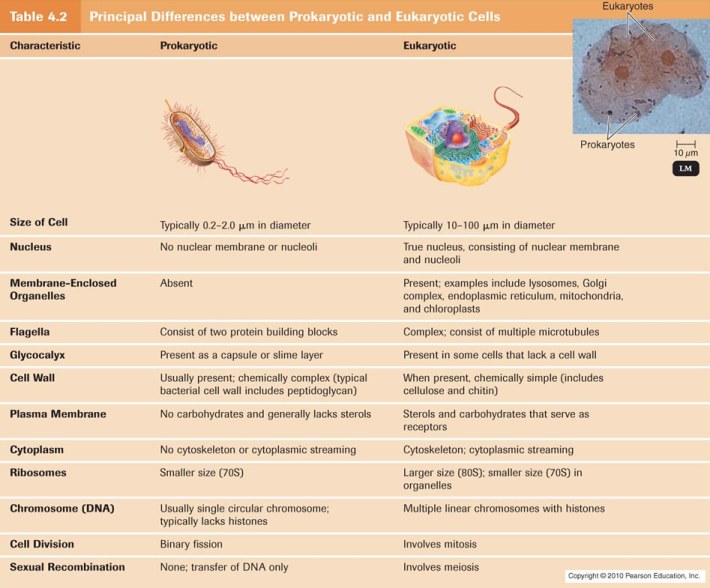
This was the first topic learnt in Biochemistry. Basically what was covered in this lesson was the definition, characteristics and functions of cells, both prokaryotic and eukaryotic. Instead of writing an entire blog explaining this, I decided to post some pictures to make the learning more visual!
Cell: Smallest unit capable of carrying out life functions. The basic unit of structure and function of all organisms.
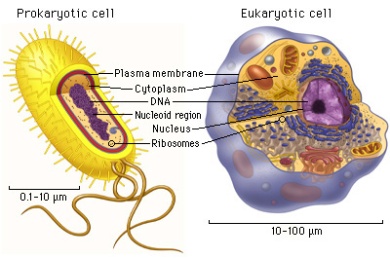
Size: Smallest free living cell: 0.5×0.5×1.6 μm (this is the dialister pneumosintes bacteria) Any cell smaller than this is unlikely to have sufficient DNA to support enzymes. This sets a LOWER LIMIT on the size of cells. On the other hand, cells cannot be too large becuase as size increases, volume increases hence surface area to volume ratio decreases, and processes such as diffusion takes too long to sustain life.
- Prokaryotic Vs Eukaryotic Cells
Eukaryotic Cell Sizes range from 10-100 μm.
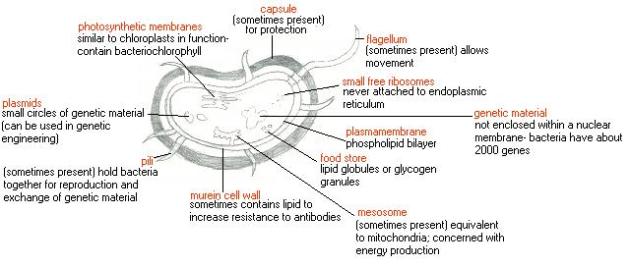
- Prokaryotic cells lack nuclei and membrane bound organelles
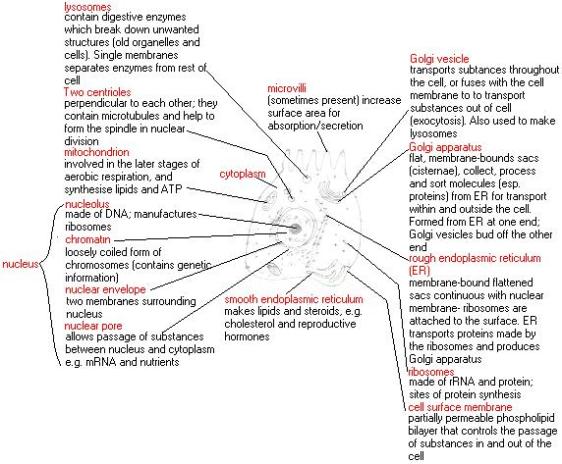
- Eukaryotic Cell:
- Eurkaryotic Cell vs Prokaryotic Cell:
Cells..here we go again!
When i saw that Cells was the first topic on the Biochemistry syllabus..i immediately thought, oh no, Cells has come back to haunt me from form 3 straight to A levels.
However, it was pretty much the same topics as A levels, just more in depth, with a few new interesting concepts. One which i found to be most interesting was Apoptosis.
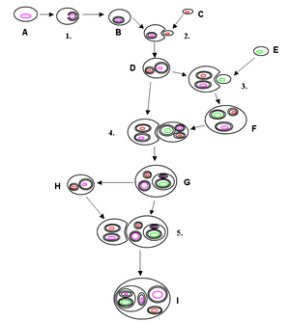
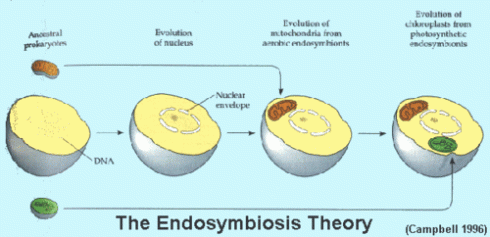
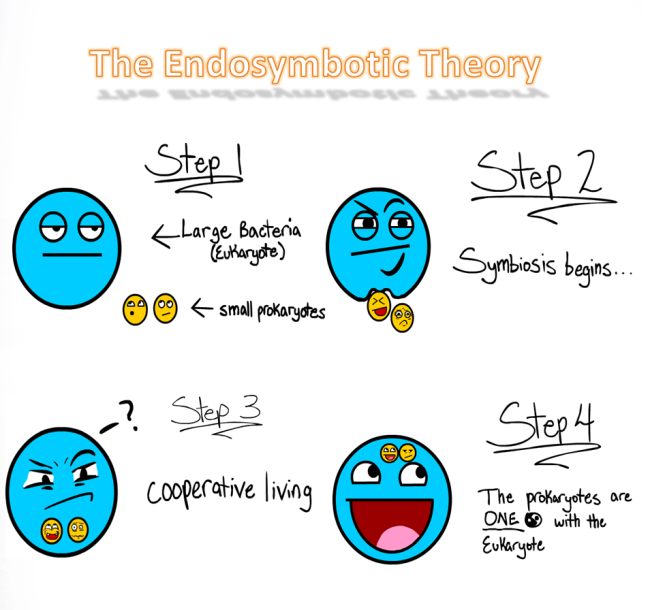
Makes you think if structures so small mimic human actions or if us humans show similar characteristics as the basic unit that supports all life. As much as i would like to go on and on about apoptosis, i’d prefer to stick to the biochem syllabus, which brings us to the Endosymbiotic Theroy.
Wait!…its actually not as complicated as it sounds, dont let the ‘big words’ fool you. The meaning is in the name itself Endo- in Symbio- Living together. Endosymbiotic theory basically states that eukaryotes originated as symbioses between separate single-celled organisms. There is overwhelming evidence in both the chloroplasts and mitochondria to prove this theory. the chloroplasts and mitochondria were once free living prokaryotic cells that were engulfed by eukaryotic cells, now living in a symbiotic relationship.
If your still confused after that explaination:
I also found a very cute video explaning this theory as a love story!
Thats all for now folks! Hope this helped you in more than one way.
References: